MedtecLIVE 2026 from 05 to 07 May in Stuttgart, Germany
We will be presenting our quality management software solutions at the MedtecLIVE trade fair from 05 to 07 May 2026 in Stuttgart, Germany. Come visit us at the joint stand of MedicalMountains in Hall 3, Booth 3-216 and find out more.
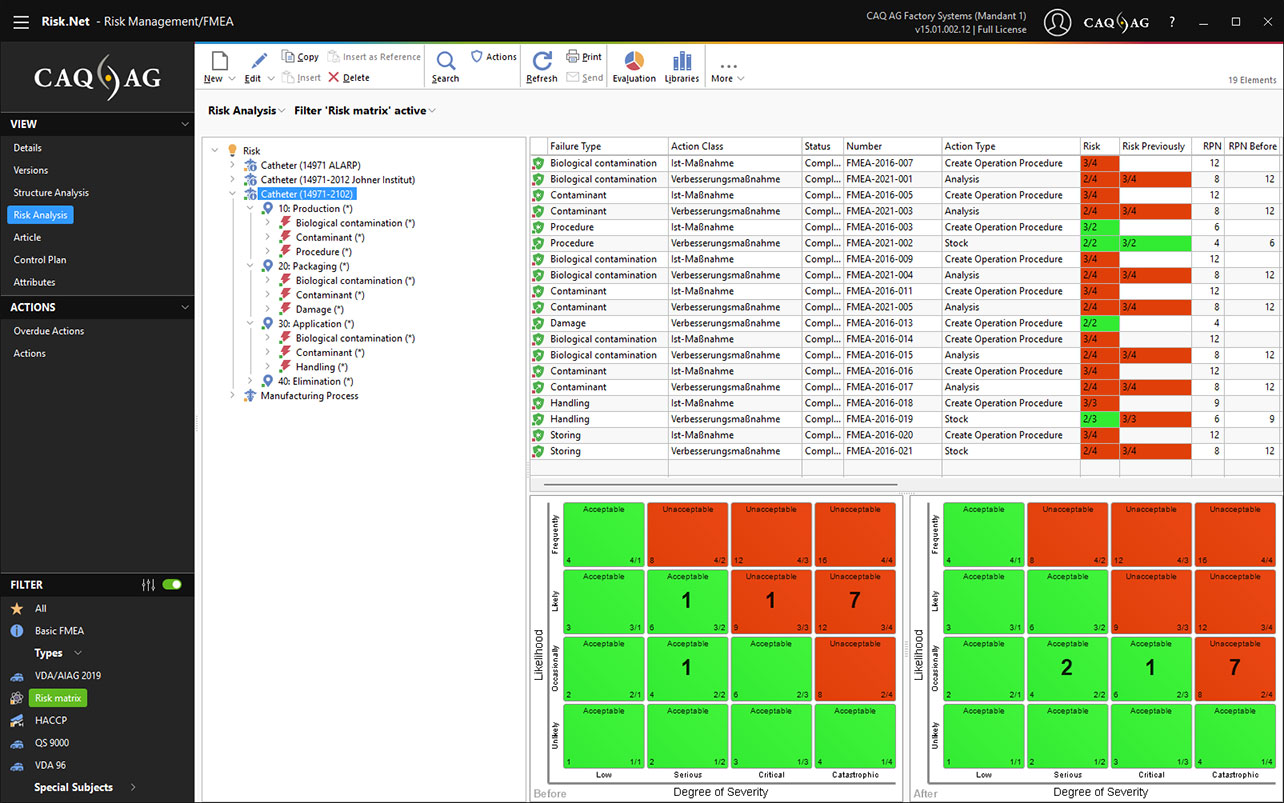 ISO 14971 Risk Matrix in the Risk Management Software Risk.Net
ISO 14971 Risk Matrix in the Risk Management Software Risk.Net
Producers and suppliers of medical technology must adhere to the strict rules and regulations of the European Medical Device Regulation (MDR) and the US Food and Drug Administration (FDA). That is why we integrated and consistently maintain powerful functions and up-to-date guidelines in our software in order to assist you in all matters relating to quality management in the area of medical technology.
It therefore comes as no surprise that, for over thirty years, many medical technology companies have put their trust in the software solutions of CAQ AG Factory Systems in order to fulfil FDA-associated framework conditions and successfully master stringent quality management and validation processes.
Visit us at the MedtecLIVE in Stuttgart and find out how our software solutions can assist you in virtually all quality-related aspects of the medical technology sector.